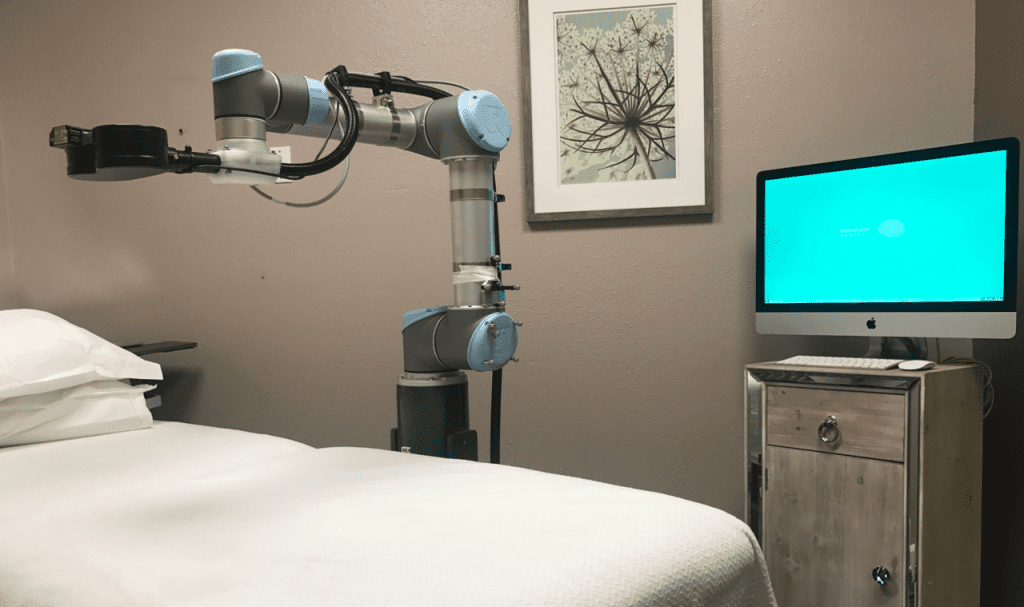
Chronic Painful Diabetic Neuropathy (PDN) poses a significant challenge for millions worldwide, causing debilitating pain and severely impacting quality of life. However, a recent breakthrough in pain management technology promises new hope for those suffering from this condition. Neuralace Medical, Inc., a pioneer in pain management solutions, has proudly announced the FDA clearance of Axon Therapy (mPNS) for the treatment of PDN. This clearance marks a significant milestone as the first-ever FDA approval of a non-invasive, magnetic peripheral nerve stimulation (mPNS) treatment for PDN.
Groundbreaking Technology
Axon Therapy represents a paradigm shift in PDN treatment. Unlike traditional methods, which often involve medications with potential side effects or invasive procedures, Axon Therapy offers a non-pharmacological, non-invasive alternative. It harnesses the power of magnetic peripheral nerve stimulation (mPNS) to deliver quick, painless relief to patients.
Efficacy and Benefits
Recent clinical trials have demonstrated the remarkable efficacy of Axon Therapy in reducing pain and improving quality of life for PDN patients. In a double-blind Multi-Center Randomized Controlled Trial (RCT) involving 71 participants, significant improvements were observed in various metrics:
– A 72.3% responder rate in the treatment group at Day 30.
– A 57.6% average reduction in Visual Analog Scale Pain Score (VAS) in the treatment group at Day 30.
– A 35% average reduction in numbness in the treatment group at Day 30.
– A 20% average reduction in Quality of Life-Diabetic Neuropathy (QoL-DN) total score at Day 30.
These results underscore the effectiveness of Axon Therapy in providing relief from PDN symptoms and enhancing overall well-being.
Patient-Centered Care
Neuralace Medical’s commitment to patient-centered care is evident in the development of Axon Therapy. By offering a non-invasive solution with minimal discomfort and no downtime, Neuralace Medical aims to improve the treatment experience for PDN patients. The clearance of Axon Therapy by the FDA signifies a significant step forward in advancing pain management strategies and providing patients with access to innovative treatment options.
Looking Ahead
With the FDA clearance of Axon Therapy, Neuralace Medical is poised to revolutionize the field of pain management. By leveraging cutting-edge technology and rigorous clinical research, Neuralace Medical continues to lead the way in developing effective solutions for chronic neuropathic pain. As awareness grows and adoption increases, Axon Therapy has the potential to transform the lives of countless individuals living with PDN, offering them renewed hope and improved quality of life.
For more information about Axon Therapy and its benefits, visit (https://